The UK, the first country to recognize the Pfizer Govt-19 vaccine, is set to begin distribution next week
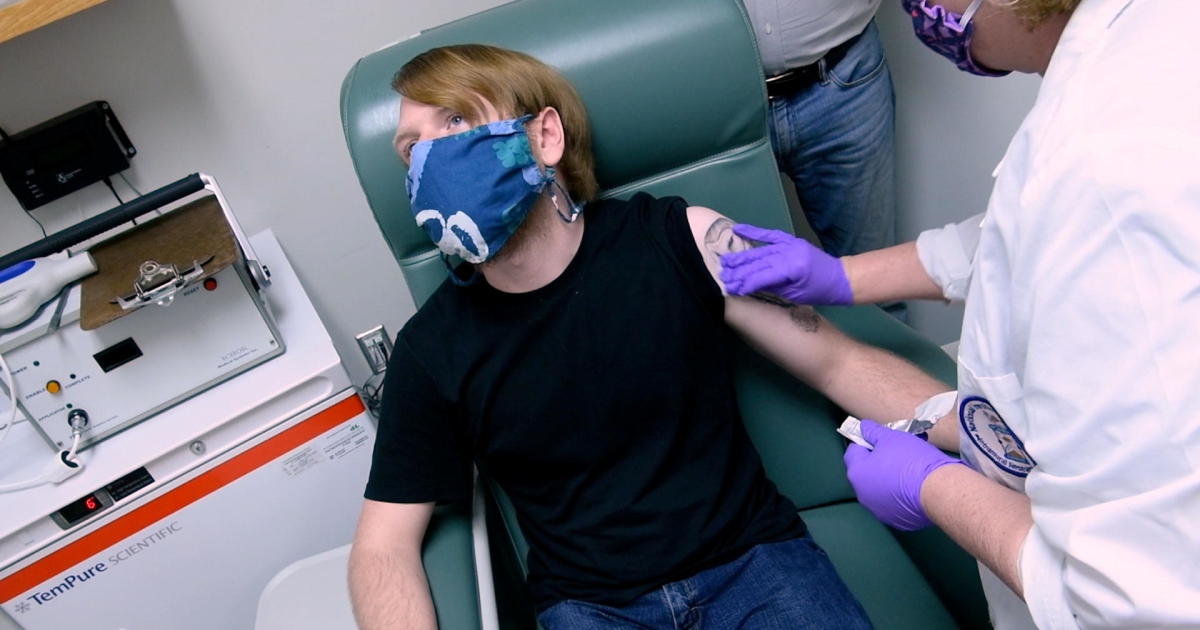
London – Britain has become the first country in the West to ratify COVID-19 Vaccine for general use. The vaccine, developed jointly by US pharmaceutical maker Pfizer and Germany’s Bioendech, has been confirmed by the UK’s Department of Health and Social Security in a report to British News Agency. Pfizer is the largest of the three major Western pharmaceutical companies To apply In both the United States and Europe for emergency use authorization.
“The government today accepted the recommendation of the Independent Pharmaceuticals and Health Products Regulatory Agency (MHRA) to approve the use of Pfizer / Bioendech’s Govt-19 vaccine,” a spokesman for the Department of Health and Social Security told the British Press. The spokeswoman concluded that the “emergency use authorization” meets strict standards of vaccine safety, quality and efficacy, following months of rigorous clinical trials and thorough analysis of MHRA experts’ data.
In order Planning in the United States, British leading health workers, caregivers, the elderly and those with basic conditions particularly vulnerable to COVID-19 complications are expected to receive the first dose of the vaccine. Pfizer’s vaccine, like the Oxford and Modern formulas, requires two doses at one-month intervals.
Britain’s Health Secretary Matt Hancock called it “fantastic news”, the UK regulator considered the first vaccine safe and vowed to start distribution from Monday. Vaccines “start in the very elderly and those in nursing homes,” he told the BBC breakfast program.
Prime Minister Boris Johnson also praised the news, confirming it in a tweet plan to launch the first levels next week.
“It’s the safety of the vaccines that will eventually allow us to recover our lives and move the economy back,” Johnson said.
Hancock, Britain’s top public health official, said Distribution logistics issues The Pfizer vaccine, which is supposed to be stored at -94 degrees Fahrenheit, said Britain would deliver its first 800,000 doses next week. There are a total of 40 million doses of Pfizer vaccine in the UK, which is enough to fully cure 20 million people.
The other two vaccines, one based in the United States Modern, Which requires a sub-zero storage, and was manufactured by a British pharmaceutical company Astrogeneka in conjunction with Oxford The university has also applied for emergency application recognition in the United States and Europe.
Data from large-scale human experiments involving tens of thousands of people, both Pfizer and Moderna, have shown that their vaccines are 95% effective in preventing COVID-19 infection. Their vaccine AstraZeneca / Oxford works differently, with up to 90% effective with a certain dose. The vaccine developed by Oxford is very cheap to produce and should be distributed as refrigeration only requires storage at room temperature.
Pfizer and Bioentech said in a joint statement on Wednesday that “this is the first emergency application approval following the global 3rd phase testing of a vaccine to help fight the infection.” Are ready to deliver. “
Pfizer chief executive Albert Burrell called the move “a historic moment in the struggle against the Covit-19s.”
None of the three leading Western-made vaccines found significant adverse effects in human testing. Experimental data show some side effects commonly associated with long-used vaccines, including soreness around the injection site, fatigue, and headache.
In China and Russia, authorities have been administering thousands of doses of COVID-19 vaccines developed by government-backed research organizations for weeks outside of testing. Unlike Western vaccination programs, neither Russia nor China carried out large-scale, international human trials before launching their non-vaccination programs with data provided for independent testing.
Hope and “Infotemic”
Logistics issues aside, epidemiologists and public health officials around the world have warned that distrust of vaccines could be another major barrier to putting the corona virus infection behind humanity.
The World Health Organization (WHO) warned on Saturday that an “infotemic” spread on social media was leaving humanity at the mercy of the new corona virus, which has killed more than 1.5 million people worldwide and nearly 271,000 in the United States alone.
“The corona virus is the first epidemic in history in which technology and social media are widely used to keep people safe, informed, productive and connected,” the WHO said in a statement. “At the same time, the technology we rely on to keep connected and informed information enables and amplifies an infotainment movement that undermines global response mitigation and infection control measures.”
The WHO has suggested that online sites and politicians and other influential people are “deliberately trying to spread misinformation” that sews distrust in scientific and healthcare organizations.
“Without proper trust and accurate information, diagnostic tests will go unused, and immunization campaigns (or campaigns promoting effective vaccines) will not meet their goals, and the virus will continue to thrive,” the WHO warned.
A CBS News Poll Suspicion was growing about the corona virus vaccine being carried out in early September, and if most voters get a vaccine this year, their first thought is that it will be rushed without adequate testing.
In a survey conducted at the end of July, it dropped from 32%, with 21% of voters nationwide saying they would soon get a vaccine at no cost. Most said they would consider it, but wait to see what happens to others before you get one.
These epidemiological pharmaceutical companies and research institutes have been found to be collaborating with governments and independent regulatory bodies, usually shortening the vaccination and approval process for many years to a very short period of time.
The pace is completely unprecedented, but the companies involved, in conjunction with external experts on infectious diseases and vaccines, have insisted throughout that the process be safely abbreviated, often rather than eliminating any steps, rather than testing and certifying the process by combining successive steps.
Hong Kong, the head of the British government’s health agency, told PTI Morgan, a UK broadcaster, on Wednesday that he would gladly show the vaccine on television next week with Morgan if they could get approval, even if they were not in the high – risk group.




